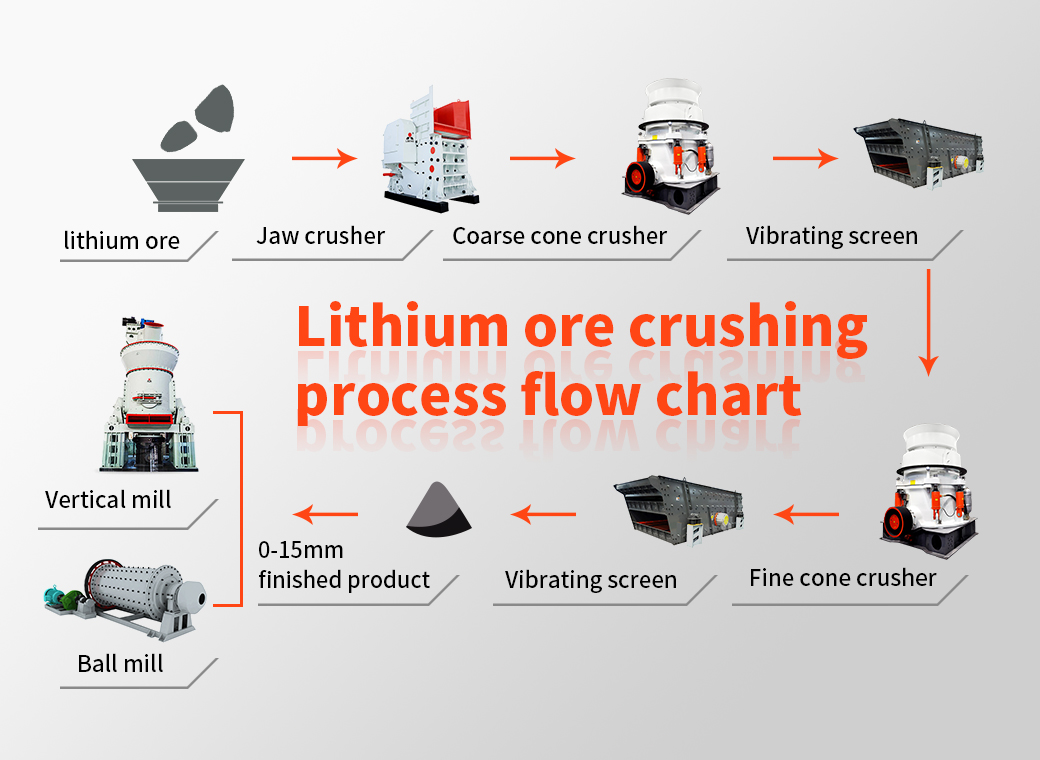
Stationary Crushers

HST Hydraulic Cone Crusher
HST series hydraulic cone crusher is combined with technology such as machinery, hydraulic pressure, electricity, automation, intelligent control, etc. , representing the most advanced crusher technology in the world. It is not only widely applied in

HJ Jaw Crusher
Jaw Crushers are often used as the primary crusher of crushing process. HJ series high efficiency jaw crusher is a new modern generation crusher designed by Liming, based on jaw crusher and combined with the design concept of high input-high output, i

CS Series Cone Crusher
According to customers requirement, our company designs high-performance crusher, CS Series High-efficiency Spring Cone Crusher. This crusher concentrates on the specialty of high-frequency, optimization of cavity-type, reasonable stroke and bases on

European Impact Crusher
By absorbing the most advanced technology in the world, PFW series European Type Impact Crusher produced by Liming Heavy Industry is the latest generation of impact crusher with first-class standard. Since the rotor is the most critical part for impa
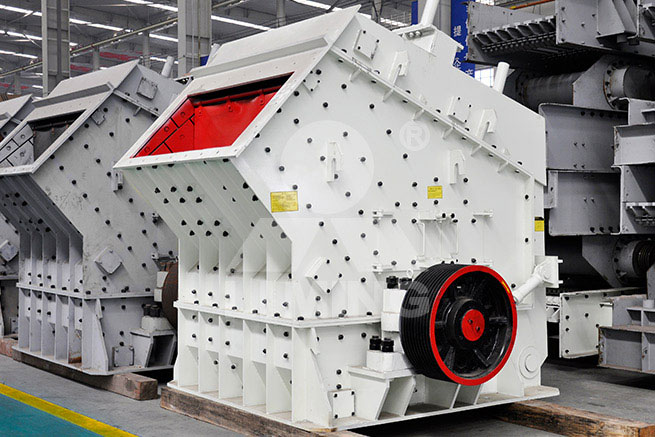
PF Impact Crusher
PF Series Impact Crusher can make final crushed products of cubic shape without tension and cracks and good grain shape. The final products can be used in railway, road construction and other industries. We supply portable, stationary impact crushing

PE Series Jaw Crusher
Jaw Crusher is ideally suitable as primary and secondary crusher for material with compression strength less than 320Mpa. Jaw Crusher is of high crushing ratio, larger capacity, well-distributed final product size, simple structure, reliable performan